How To Use A Spectrophotometer
ii.1.v: Spectrophotometry
- Page ID
- 1431
Spectrophotometry is a method to measure how much a chemical substance absorbs calorie-free by measuring the intensity of light as a beam of light passes through sample solution. The basic principle is that each chemical compound absorbs or transmits light over a certain range of wavelength. This measurement tin also be used to measure the amount of a known chemical substance. Spectrophotometry is one of the most useful methods of quantitative analysis in various fields such equally chemistry, physics, biochemistry, material and chemical engineering and clinical applications.
Introduction
Every chemical compound absorbs, transmits, or reflects light (electromagnetic radiation) over a sure range of wavelength. Spectrophotometry is a measurement of how much a chemical substance absorbs or transmits. Spectrophotometry is widely used for quantitative assay in diverse areas (e.g., chemistry, physics, biological science, biochemistry, material and chemical applied science, clinical applications, industrial applications, etc). Any application that deals with chemical substances or materials can utilize this technique. In biochemistry, for case, information technology is used to determine enzyme-catalyzed reactions. In clinical applications, it is used to examine blood or tissues for clinical diagnosis. There are as well several variations of the spectrophotometry such as atomic assimilation spectrophotometry and atomic emission spectrophotometry.
A spectrophotometer is an instrument that measures the amount of photons (the intensity of low-cal) absorbed subsequently it passes through sample solution. With the spectrophotometer, the amount of a known chemical substance (concentrations) can also be adamant by measuring the intensity of calorie-free detected. Depending on the range of wavelength of light source, it can be classified into two different types:
- UV-visible spectrophotometer: uses light over the ultraviolet range (185 - 400 nm) and visible range (400 - 700 nm) of electromagnetic radiation spectrum.
- IR spectrophotometer: uses light over the infrared range (700 - 15000 nm) of electromagnetic radiations spectrum.
In visible spectrophotometry, the absorption or the manual of a certain substance tin can be determined by the observed color. For instance, a solution sample that absorbs light over all visible ranges (i.e., transmits none of visible wavelengths) appears black in theory. On the other mitt, if all visible wavelengths are transmitted (i.eastward., absorbs cipher), the solution sample appears white. If a solution sample absorbs red light (~700 nm), it appears green because dark-green is the complementary colour of red. Visible spectrophotometers, in do, use a prism to narrow down a certain range of wavelength (to filter out other wavelengths) so that the particular beam of light is passed through a solution sample.
Devices and mechanism
Effigy 1 illustrates the basic structure of spectrophotometers. It consists of a low-cal source, a collimator, a monochromator, a wavelength selector, a cuvette for sample solution, a photoelectric detector, and a digital brandish or a meter. Detailed machinery is described below. Figure 2 shows a sample spectrophotometer (Model: Spectronic 20D).
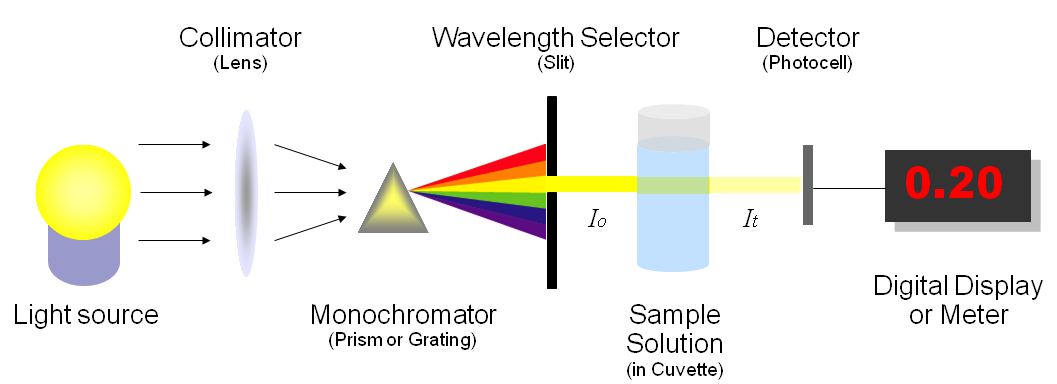
A spectrophotometer, in general, consists of two devices; a spectrometer and a photometer. A spectrometer is a device that produces, typically disperses and measures light. A photometer indicates the photoelectric detector that measures the intensity of light.
- Spectrometer: Information technology produces a desired range of wavelength of light. Start a collimator (lens) transmits a directly beam of light (photons) that passes through a monochromator (prism) to split information technology into several component wavelengths (spectrum). Then a wavelength selector (slit) transmits only the desired wavelengths, as shown in Figure 1.
- Photometer: Subsequently the desired range of wavelength of light passes through the solution of a sample in cuvette, the photometer detects the corporeality of photons that is absorbed and and so sends a signal to a galvanometer or a digital display, every bit illustrated in Effigy one.

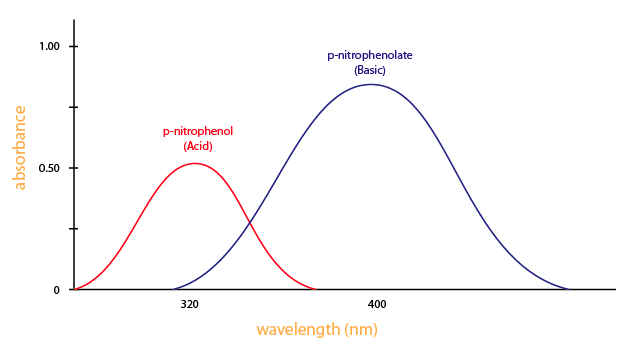
You demand a spectrometer to produce a variety of wavelengths considering unlike compounds absorb best at different wavelengths. For instance, p-nitrophenol (acid form) has the maximum absorbance at approximately 320 nm and p-nitrophenolate (basic form) absorb best at 400nm, as shown in Figure 3.
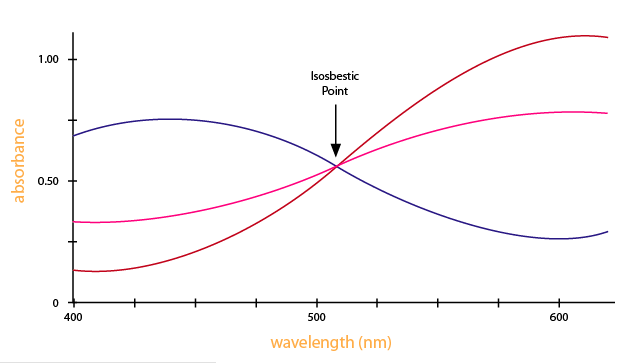
Looking at the graph that measures absorbance and wavelength, an isosbestic point tin also be observed. An isosbestic indicate is the wavelength in which the absorbance of 2 or more than species are the same. The appearance of an isosbestic point in a reaction demonstrates that an intermediate is NOT required to form a product from a reactant. Figure iv shows an example of an isosbestic point.
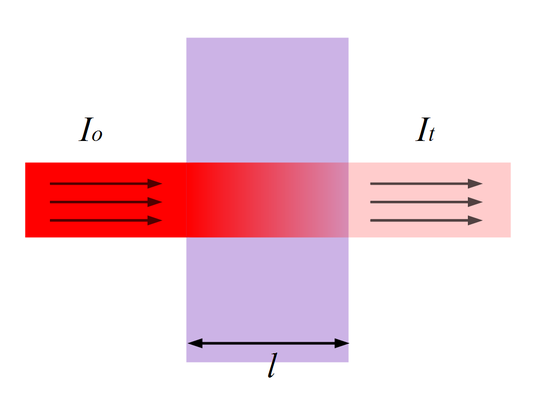
Referring dorsum to Figure 1 (and Effigy 5), the amount of photons that goes through the cuvette and into the detector is dependent on the length of the cuvette and the concentration of the sample. In one case you know the intensity of low-cal subsequently it passes through the cuvette, you lot tin chronicle information technology to transmittance (T). Transmittance is the fraction of low-cal that passes through the sample. This tin can be calculated using the equation:
\(Transmittance (T) = \dfrac{I_t}{I_o}\)
Where It is the light intensity after the beam of light passes through the cuvette and Io is the light intensity earlier the beam of light passes through the cuvette. Transmittance is related to absorption by the expression:
\(Absorbance (A) = - log(T) = - log(\dfrac{I_t}{I_o})\)
Where absorbance stands for the amount of photons that is absorbed. With the corporeality of absorbance known from the above equation, you tin can make up one's mind the unknown concentration of the sample by using Beer-Lambert Law. Figure v illustrates transmittance of low-cal through a sample. The length \(fifty\) is used for Beer-Lambert Law described below.
Beer-Lambert Police
Beer-Lambert Law (also known as Beer'south Law) states that there is a linear relationship between the absorbance and the concentration of a sample. For this reason, Beer'southward Police tin can merely be applied when in that location is a linear relationship. Beer'south Law is written as:
\(A = \epsilon{lc}\)
where
- \(A\) is the measure out of absorbance (no units),
- \(\epsilon\) is the molar extinction coefficient or molar absorptivity (or absorption coefficient),
- \(l\) is the path length, and
- \(c\) is the concentration.
The molar extinction coefficient is given equally a constant and varies for each molecule. Since absorbance does not carry whatsoever units, the units for \(\epsilon\) must cancel out the units of length and concentration. As a event, \(\epsilon\) has the units: L·mol- ane·cm- i. The path length is measured in centimeters. Because a standard spectrometer uses a cuvette that is i cm in width, \(50\) is always assumed to equal one cm. Since assimilation, \(\epsilon\), and path length are known, nosotros can summate the concentration \(c\) of the sample.
Example 1
Guanosine has a maximum absorbance of 275 nm. \(\epsilon_{275} = 8400 Thou^{-1} cm^{-1}\) and the path length is i cm. Using a spectrophotometer, you find the that \(A_{275}= 0.seventy\). What is the concentration of guanosine?
Solution
To solve this problem, you must use Beer'southward Law.
\[A = \epsilon lc \]
0.seventy = (8400 Grand-one cm-1)(1 cm)(\(c\))
Next, divide both side by [(8400 K-1 cm-1)(1 cm)]
\(c\) = 8.33x10-5 mol/L
Case 2
At that place is a substance in a solution (4 g/liter). The length of cuvette is 2 cm and just fifty% of the certain light axle is transmitted. What is the absorption coefficient?
Solution
Using Beer-Lambert Law, we can compute the assimilation coefficient. Thus,
\(- \log \left(\dfrac{I_t}{I_o} \right) = - \log(\dfrac{0.five}{i.0}) = A = {8} \epsilon\)
Then we obtain that
\(\epsilon\) = 0.0376
Instance three
In example 2 above, how much is the beam of light is transmitted when viii chiliad/liter ?
Solution
Since nosotros know \(\epsilon\), we can calculate the manual using Beer-Lambert Police. Thus,
\(\log(1) - \log(I_t) = 0 - \log(I_t)\) = 0.0376 10 8 ten 2 = 0.6016
\(\log(I_t)\) = -0.6016
Therefore, \(I_t\) = 0.2503 = 25%
Example four
In example ii above, what is the tooth assimilation coefficient if the molecular weight is 100?
Solution
It can simply obtained past multiplying the absorption coefficient by the molecular weight. Thus,
\(\epsilon\) = 0.0376 x 100 = 3.76 L·mol- 1·cm- i
Example 5
The absorption coefficient of a glycogen-iodine complex is 0.20 at light of 450 nm. What is the concentration when the transmission is forty % in a cuvette of 2 cm?
Solution
Information technology can as well be solved using Beer-Lambert Law. Therefore,
\[- \log(I_t) = - \log(0.4) = 0.20 \times c \times two\]
Then \(c\) = 0.9948
References
- Atkins, Peter and Julio de Paula. Physical Chemical science for the Life Sciences. New York: Oxford University Press, 2006.
- Chang, Raymond. Concrete Chemistry for the Biosciences. USA: University Science Books, 2005.
- Gore, Michael. Spectrophotometry & Spectrofluorimetry. New York: Oxford University Printing, 2000.
- Price, Nicholas and Dwek, Raymond and Wormald, Mark. Principles and Bug in Physical Chemistry for Biochemists. R. G. Ratcliffe. New York: Oxford University Printing, 1997.
- Irwin H. Segel, Biochemical Calculations (How to Solve Mathematical Bug in General Biochemistry), 2nd edition, John Wiley & Sons, 1975
- http://world wide web.nist.gov/pml/div685/grp03/spectrophotometry.cfm
Contributors and Attributions
- Kevin Vo (UCD)
How To Use A Spectrophotometer,
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Kinetics/02:_Reaction_Rates/2.01:_Experimental_Determination_of_Kinetics/2.1.05:_Spectrophotometry
Posted by: velasquezchricand.blogspot.com


0 Response to "How To Use A Spectrophotometer"
Post a Comment